Ionization is one of the fundamental and important concepts in chemistry. It describes the separation of ionic compounds into separate ions when they are dissolved in a solvent. This has great significance in many fields of science, from chemistry, biology to medicine. In this article, we will learn about dissociation in detail, classify strong electrolytes and weak electrolytes, as well as the factors that affect this process.
What is an electrolyte?
An electrolyte is a chemical substance that, when dissolved in a solvent, especially water, separates into free ions. These substances are capable of conducting electricity when dissolved or molten. When an electrolyte is dissolved, its molecules become polarized and separate into positively charged ions (cations) and negatively charged ions (anions).
A typical example of an electrolyte is table salt (sodium chloride – NaCl). When table salt is dissolved in water, the NaCl molecules separate into Na+ ions (positive sodium) and Cl- ions (negative chloride). This separation is called dissociation.
Electrolytes can be divided into two main types: strong electrolytes and weak electrolytes.
Classification of strong electrolytes and weak electrolytes
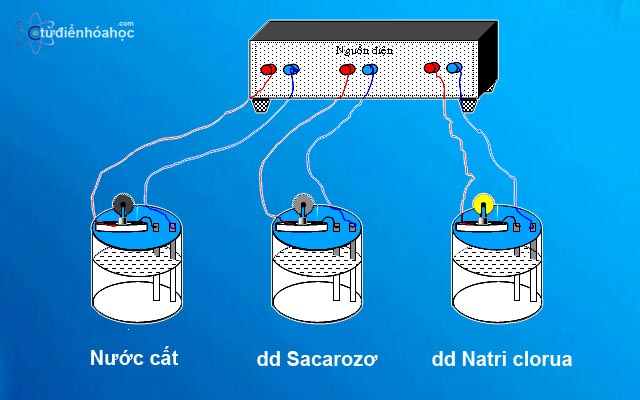
Strong electrolyte
Strong electrolytes are chemical substances that, when dissolved in a solvent, especially water, undergo complete ionization. That is, when dissolved, almost all of the molecules of the substance become polarized and separate into free ions. This means that when strong electrolytes dissolve in water, very little or none of the original molecules remain.
Some examples of strong electrolytes:
- Strong inorganic acids: Hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3).
- Strong inorganic bases: Sodium hydroxide (NaOH), potassium hydroxide (KOH).
- Salts soluble in water: Sodium chloride (NaCl), potassium nitrate (KNO3), magnesium sulfate (MgSO4).
Strong electrolytes have a degree of dissociation that is nearly 100%, meaning that when dissolved, they dissociate into ions almost completely.
Weak electrolyte
Weak electrolytes are chemical substances that, when dissolved in a solvent, especially water, will have only a small portion of the molecules polarized and separated into free ions. That is, when dissolved, only a small portion of the molecules of the substance will be ionized, the majority still exists in the original molecular form.
Some examples of weak electrolytes:
- Acetic acid (CH3COOH)
- Ammonia (NH3)
- Carbonic acid (H2CO3)
- Hypochlorous acid (HOCl)
Weak electrolytes have a degree of dissociation less than 100%, usually between 1 and 5%. This means that when dissolved, only a small fraction of the substance’s molecules will dissociate into ions, while the majority remain as the original molecule.
Comparison of strong electrolytes and weak electrolytes
The fundamental difference between strong electrolytes and weak electrolytes lies in the rate at which they dissociate into free ions when dissolved in a solvent.
A strong electrolyte will have almost complete dissociation (about 100%), meaning that almost all of the molecule will be dissociated into free ions. Meanwhile, a weak electrolyte will have only a small portion of the molecule dissociated into ions (from 1 to 5%), the majority still remaining in the original molecular form.
This leads to some fundamental differences between the two types of electrolytes in terms of properties and applications. For example, strong electrolytes are generally better conductors of electricity and their solutions change pH more strongly than weak electrolytes.
Arrhenius theory of electrolysis
The theory of electrolytic dissociation was first proposed in 1887 by the Swedish chemist Svante Arrhenius. This theory explains the polarization and separation of free ions when electrolytes are dissolved in solvents, especially water.
According to Arrhenius, when an electrolyte such as a salt, acid or base is dissolved in water, the molecules of the substance will be polarized and separate into positively charged ions (cations) and negatively charged ions (anions). This process is called dissociation.
For example, when table salt (sodium chloride – NaCl) is dissolved in water, the NaCl molecules become polarized and separate into Na+ ions (positive sodium) and Cl- ions (negative chloride):
NaCl → Na+ + Cl-
Such dissociation makes the solution capable of conducting electricity, since the free ions can move and conduct current.
According to Arrhenius, the degree of dissociation of a substance depends on:
- Molecular structure of electrolytes.
- The attraction between the ions forms.
- Properties of solvents.
For example, strong electrolytes such as hydrochloric acid (HCl) or sodium hydroxide (NaOH) have almost complete dissociation (100%) when dissolved in water. Meanwhile, weak electrolytes such as acetic acid (CH3COOH) have only a small fraction of the molecule dissociated into ions (dissociation degree of about 1-5%).
The Arrhenius theory of electrolysis explained many phenomena related to the properties of electrolyte solutions, such as electrical conductivity, acid-base properties, redox reactions, etc. This was one of the important achievements of chemistry in the early 20th century.
Bronsted – Lowry theory of electrolytic dissociation
Although the Arrhenius theory explained many phenomena related to electrolysis, it still had some limitations. In 1923, chemists Johannes Bronsted and Thomas Lowry proposed a new view of electrolysis, called the Bronsted-Lowry theory.
According to the Bronsted – Lowry theory, dissociation is defined as the exchange of protons between substances. Specifically:
- A substance that can give up protons is called an acid.
- A substance that can accept a proton is called a base.
- Dissociation occurs when an acid exchanges protons with a base.
For example, when hydrochloric acid (HCl) is dissolved in water, it exchanges protons with water (H2O) and produces hydrogen ions (H3O+) and chloride ions (Cl-):
HCl + H2O ⇌ H3O+ + Cl-
In which, HCl is an acid because it gives out protons, and H2O is a base because it accepts protons.
The Bronsted-Lowry theory extends the scope of electrolysis compared to the Arrhenius theory. It not only explains the dissociation of acids, bases, and salts, but is also applicable to other proton transfer reactions in chemistry.
For example, when NH3 (ammonia) dissolves in water, it accepts protons from the water and produces ammonium ions (NH4+) and hydroxide ions (OH-):
NH3 + H2O ⇌ NH4+ + OH-
Thus, NH3 is a base because it accepts protons, and H2O is an acid because it gives up protons.
The Bronsted-Lowry theory expanded and added many important concepts in chemistry, such as conjugate acid-base pairs, pH, pKa, etc. This is one of the important achievements of modern chemistry.
Degree of dissociation
Degree of ionization is an important parameter to describe the degree of dissociation of electrolytes when dissolved in solvents.
Degree of dissociation is denoted by α (alpha) and is defined as the ratio of the mole fraction of free ions to the total mole fraction of the electrolyte initially dissolved in the solvent.
Formula for calculating degree of dissociation:
α = (number of moles of free ions) / (total number of moles of initial electrolyte)
For example, when 1 mole of NaCl is dissolved in water, if 0.9 mole of NaCl is dissociated into Na+ and Cl- ions, then the degree of dissociation of NaCl will be:
α = 0.9 / 1 = 0.9 = 90%
Thus, the degree of dissociation of NaCl is 90%, meaning that 90% of the original NaCl molecules have dissociated into free ions.
The degree of dissociation of electrolytes has the following characteristics:
- Strong electrolytes have a degree of dissociation of nearly 100%.
- Weak electrolytes have low degrees of dissociation, usually only 1 to 5%.
- The degree of dissociation depends on temperature, concentration and properties of the solvent.
Dissociation is an important parameter because it directly affects many properties of the solution, such as electrical conductivity, pH, redox effects, etc.
Factors affecting electrolysis
The dissociation of substances depends on many factors, including:
1. Nature of electrolytes
- Strong electrolytes have a high degree of dissociation, almost 100%.
- Weak electrolytes have low degrees of dissociation, usually only 1 to 5%.
2. Properties of solvents
- Highly polar solvents such as water will increase the degree of dissociation of the solute.
- Solvents with low polarity such as benzene will decrease the degree of dissociation.
3. Concentration of solution
- At high concentrations, the dissociation of weak electrolytes will be inhibited due to the common ion effect.
- At low concentrations, the dissociation of weak electrolytes will increase.
4. Temperature
- Dissociation usually increases with temperature because it stimulates ion dissociation.
- However, there are some cases where the degree of dissociation decreases with temperature due to the phenomenon of equilibrium shifting.
These factors act and interact with each other to determine the degree of dissociation of a substance in a particular solution.
Applications of electrolysis
Electrolysis is not only a theoretical concept but also has many practical applications in life and industry. Below are some common applications of electrolysis:
1. Conductivity in solution
- Electrolysis helps the solution conduct electricity, turning water into a good conductive solvent.
- Applications in the production of electrolyte solutions, electrode solutions, etc.
2. Chemical analysis
- Electrolysis helps determine the properties of substances in solution, thereby applying to chemical analysis.
- Applications in acid-base titration, ion analysis, etc.
3. Biochemistry and medicine
- Electrolysis helps to understand chemical reactions in the body, which has applications in medicine and biochemistry.
- Applications in the study of enzyme activity, chemical reactions in the body, etc.
4. Environmental technology
- Electrolysis helps treat wastewater, recycle solutions, and treat environmental pollution.
- Applications in water treatment technology, industrial waste treatment, etc.
5. Material technology
- Electrolysis helps create new types of materials, from electrolytes to conducting polymers.
- Applications in the production technology of conductive materials, smart materials, etc.
Applications of electrolysis not only help us better understand the properties of chemicals but also open many new doors in research and technological applications.
Examples of strong electrolytes and weak electrolytes
To better understand dissociation, let’s look at some examples of strong and weak electrolytes:
Example of a strong electrolyte: NaCl (Sodium chloride)
- When NaCl is dissolved in water, it completely dissociates into sodium ions (Na+) and chloride ions (Cl-).
- The degree of dissociation of NaCl is almost 100% due to the creation of strong free ions.
Example of a weak electrolyte: CH3COOH (Acetic acid)
- When CH3COOH is dissolved in water, only a small fraction of the CH3COOH molecules will dissociate into acetate ions (CH3COO-) and protons.
- The degree of dissociation of CH3COOH is low, about 1-5%, because only a small fraction of the molecules are dissociated.
These examples show the clear difference between strong electrolytes and weak electrolytes based on their dissociation and ability to dissociate ions.
Meaning of electrolysis
Electrolysis plays an important role in chemistry and related sciences. The significance of electrolysis includes:
- Helps understand the properties of chemicals when dissolved in solvents.
- Applied to many chemical analysis methods and chemical structure determination.
- Opens up many applications in industry, medicine, environment, materials, etc.
- Plays an important role in research and development of new technologies.
Electrolysis is not only an abstract concept but also an important tool to help people better understand the world around them and apply it to real life.
Notes when doing exercises on electrolysis
When doing exercises on electrolysis, keep in mind the following points to get good results:
- Understand the definition and properties of strong electrolytes and weak electrolytes.
- Know how to calculate the degree of dissociation and apply it to specific exercises.
- Identify the factors affecting dissociation and understand the mechanism of ion dissociation.
- Practice many exercises to master knowledge and skills related to electrolysis.
- Read more reference books and learn about the applications and significance of electrolysis in real life.
By applying the above notes, you will have the opportunity to grasp and apply knowledge about electrolysis effectively in your studies and research.
Conclude
Above is an overview of electrolysis in chemistry, from definition, classification, theory to its applications and significance. Electrolysis is not only a basic concept but also an important foundation to help us better understand the properties of chemical substances and apply it in practice.
Hopefully this article has helped you better understand dissociation and how to apply this knowledge to your studies and research. Continue to explore and apply dissociation in chemistry to open up many new opportunities in the future. Good luck!
For any questions, please send to Hotline 09633458xxx or email address [email protected] for clarification. Best regards!
Tuyên bố miễn trừ trách nhiệm: sesua.vn là website tổng hợp kiến thức từ nhiều nguồn,Vui lòng gửi email cho chúng tôi nếu có bất cứ vi phạm bản quyền nào! Xin cám ơn!
- Come out là gì?
- Ảnh Meme Là Gì?
- Phương pháp cân bằng phương trình hóa học cho phản ứng: P + HNO3 → H3PO4 + NO2 + H2O
- Công ty đại chúng công bố thông tin về ngày đăng ký cuối cùng dự kiến thực hiện quyền cho cổ đông hiện hữu để tham dự họp Đại hội đồng cổ đông khi nào?
- Ý nghĩa của hoa lưu ly trong tình yêu, đời sống chi tiết nhất














